There's little doubt that you've had some experience with static electricity. That annoying "shock" you feel after you walk across the carpet and touch the doorknob, for instance. Or that equally annoying thing your hair does when you pull a wool hat quickly off your head. But what is static electricity, and why does it cause these types of events to occur?
In this section, you'll not only learn about static electricity, but also about a really cool experiment regarding static electricity that you can do with a simple device you'll make called an electroscope. You'll use the electroscope to detect electrical charge.
So What Seems to Be the Problem?
The problem you'll attempt to solve is how well certain objects give up their electrons to other objects. How does that relate to static electricity, you ask?
In the wonderful world of science, there are protons, neutrons, and electrons. Protons and neutrons are tiny particles contained within the nucleus of an atom.
Protons and neutrons are tiny particles contained within the nucleus of an atom. Electrons are even tinier particles that orbit around the nucleus.
An atom, if you'll recall, is the smallest piece possible of an object. A bar of silver, for instance, could be divided in half, then half again, and again and again and again, until there is a piece so small that if it were to be divided, it would no longer be silver. That very last piece is called an atom.
In the middle of each atom is a nucleus. That's where the protons and neutrons hang out. Electrons, on the other hand, are even smaller than protons and neutrons, and orbit around the nucleus of the atom.
Electrical Charges
Protons, neutrons, and electrons are very different from one another. In terms of this experiment, the way that they're different from one another concerns their electrical charges.
Protons have a positive (+) charge. Electrons have a-you guessed it-negative (-) charge, and neutrons, as their name suggests, have no charge. When an atom contains the same number of protons and electrons, the atom has no overall charge, but is neutral. That's because the positive charge of a proton is equal to the negative charge of an electron. Put together, one cancels out the other.
While protons and neutrons stick closely together within the nucleus of an atom, electrons are like free spirits who can't stay still. They move around about the nucleus, and sometimes bail out on the atom altogether and move to a different atom.
When this happens, it puts the electric charge of the atom out of balance. Remember, a neutral atom must contain the same number of protons and electrons. When electrons jump ship and move to another atom, the balance is lost.
An atom that loses electrons then has a positive charge because it contains more protons than electrons. The atom that gains the electrons has more negative than positive particles, so it has a negative charge.
Just to make things a bit more confusing, an atom that has either a positive or negative charge is no longer called an atom. It's now known as an ion.
Some materials hold their electrons very closely, not allowing the electrons to move through them well. These materials are known as insulators. Materials that do allow their electrons to move through them easily are called conductors. Cloth is a good insulator, while metals generally are good conductors.
Electrons and Static Electricity
It's important to realize that electrons moving from place to place is not an unusual occurrence. It happens all the time, whenever two objects rub together. When there is a lot of contact between two objects, a lot of electrons get transferred, and the amount of charge builds up. Static electricity, simply put, is nothing more than an imbalance of positive and negative charges.
The next idea to understand is that opposite charges attract, while charges that are the same repel each other. When you pull a hat off your head and your hair does that weird, standing-up-straight thing, it's because of this attract-and-repel rule.
Electrons that were in your hair rub off onto the hat. When you remove the hat, the electrons go with it, leaving your hair with only a positive charge. Each little hair tries to get away from its same-charged neighbor, resulting in that flyaway look. Static electricity is an imbalance of positive and negative charges.
All right, enough explanation. It's time to get down to business. In this science fair project, you'll attempt to discover which objects most readily release their electrons, allowing a charge to result. Remember that some objects (insulators) do not give up electrons as easily as others (conductors).
If you want to, you can use the title of this section, "What Materials Conduct Static Electricity Best?" as your project title. Or you could call your project one of the titles suggested below:
- How Does Transferring Electrons Change a Charge?
- Understanding Static Electricity
- Using an Electroscope to Detect Electrical Charge
Once you understand the concept of static electricity, you can think about everyday applications of this project.
What's the Point?
Now you know why your hair stands straight up when you pull a wool hat off your head. So what?
Understanding electrical charges is important for many reasons. It helps you to understand how the world works, and why certain events occur.
Did you ever notice that static electricity shocks occur mostly in the winter? And that even if you don't pull a hat off your head, your hair tends to be a little flyaway whenit's cold outside?
This is because the air in the winter generally is very dry. Summer air normally is more humid, meaning that it contains more moisture. What happens is that the water in the summer air helps electrons to move off your body more quickly. Water is a good conductor.
Because the electrons move off your body, you don't build up a big charge. In the winter, however, when the electrons stay on your body, you build up a negative charge. So when you walk across the rug, the electrons from the rug move onto, and build up on, your body. When you touch the doorknob, the electrons jump from you to the metal knob (remember that metal is a good conductor), causing you to feel a shock.
Charged objects emit invisible electric force fields that surround them. The strength of these fields varies depending on many factors. Way back in the 1780s, a scientist named Charles Coulomb described and investigated these force fields. The formula he came up with for figuring out the strength of the fields is called Coulomb's Law.
In this experiment, you'll use the electroscope you'll make to determine which objects best build up and conduct electrical charges. The control you'll use is uncharged aluminum foil, and the variables you'll use will be other common objects, all of which are normally found around a house.
What Do You Think Will Happen?
You should have a basic but sound understanding now of how static electricity occurs, and what happens when it does. Before you venture a hypothesis, though, you'll need to understand how the experiment works.
In this experiment, you'll test how well certain objects transfer electrons, using aluminum foil as the detector.
During the experiment, you'll transfer electrons from one object to another by rubbing a flexible, plastic ruler on different materials. The ruler will serve as the conductor of the electrons onto the aluminum foil.
When you rub the ruler on different objects, it will either pick up electrons from the object, or it will pass some of its electrons onto the object. If the ruler picks up electrons from the material on which it's rubbed, it will have a negative charge. If the ruler gives off electrons, it will have a positive charge.
To come up with a hypothesis, venture a guess on which materials you think are most likely to transfer electrons onto the ruler. Do you have a hunch that some might be more effective at doing that than others?
Go ahead and take your best guess. Then we'll get started on the experiment.
Materials You'll Need for This Project
An electroscope is a device that detects electrical charge. There are many types of electroscopes, some complicated models that you could buy from a scientific supplier, and some simple ones you can make yourself.
You'll need only four materials to make a simple electroscope. They are:
- One small cup (glass or paper)
- One plastic drinking straw with flexible end
- Tape
- Aluminum foil
The other materials you'll need for the experiment are those mentioned in the previous section:
- A flexible plastic ruler
- Wool
- Silk
- Cotton
- Newspaper
- Carpeting
If you can't get the materials specified above, feel free to substitute something you have on hand. Part of the beauty of this experiment is that you probably won't have to buy anything. If you do, it shouldn't cost more than a dollar or two to get what you need.
This experiment will work best on a day that is cool and dry, rather than warm and humid. Conducting it inside the house on a day when the heat is turned on would give you favorable conditions.
Conducting Your Experiment
Before you begin the actual experiment, you'll need to build your electroscope. Don't worry-"building" the electroscope really doesn't require any building.
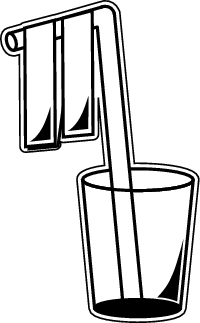
Follow these steps:
1. Place a straw into a small cup. The flexible part of the straw should be at the top, and the straw should be bent.
2. Cut two small strips of aluminum foil, about 21/2 inches (6 cm) long, and 1/2 inch (1 cm) wide.
3. Tape the strips onto the bent part of the straw, so that they're next to each other, but not touching. They should hang straight down from the bent arm of the straw.
Now your electroscope is ready and you can move on to the next steps of the experiment.
4. Rub the ruler onto the piece of wool, and then bring the ruler close to the aluminum foil, without actually touching it. Rubbing the ruler with silk will remove electrons from the ruler, giving the ruler a positive charge. Wool, however, will transfer electrons onto the ruler, resulting in a negative charge. You can test any material to see if it takes or gives electrons.
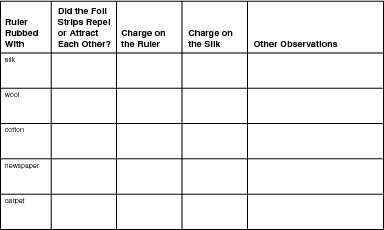
5. Record your observations, noting which material you used and the reaction you observed with the foil strips.
6. Repeat steps 4 and 5 with each of the other materials. Note which materials result in the aluminum foil being attracted to the ruler, and which make the foil and ruler repel one another. Realize that the neutral foil strips may at first be attracted to the charged ruler, and then within a second or two repel the ruler. This would occur because the foil strips picked up electrons from the ruler, and both have the same charge.
7. Repeat the entire experiment three times, but leave an hour in between each repetition to let the foil strips regain some stability. Try to make sure the surroundings for the experiment are the same for each trial.
Be sure to keep accurate notes on what you observe with each material.
Keeping Track of Your Experiment
You can use the following chart to keep track of your observations. Or you could make your own, similar chart, if you prefer.
Putting It All Together
Once you've tested a variety of materials to see which conduct static electricity the best, you'll have a good idea about which materials release electrons and which take electrons.
Check your observations carefully to see which materials reacted best with the aluminum foil.
Further Investigation
If you enjoyed this project, there are many more experiments you can do to explore static electricity and the transfer of electrons.
A fun experiment would be to test the carpeting found in different rooms of your house. See how a ruler rubbed onto the carpet of one room reacts with the aluminum foil, compared with that in other rooms.
Another fun experiment is to see if you can build up enough static electricity on a balloon or other object to "bend" water. Simply turn on the water so that it's coming out of the faucet in a very thin stream.
Rub a balloon onto a wool sweater, stuffed animal, or other furry object, and then hold it close to the stream of water. The water, which is neutral, will be attracted to the charged balloon, and will "bend" toward it.
Learn about electricity in this "shocking" science experiment!
In this hands-on science project, pupils build their own electroscopes to determine what materials act as the best conductor of static electricity.
Looking for project-based learning?
Check out our collection of hands-on projects that combine math, ELA, and science concepts with 21st Century and social-emotional skills!




